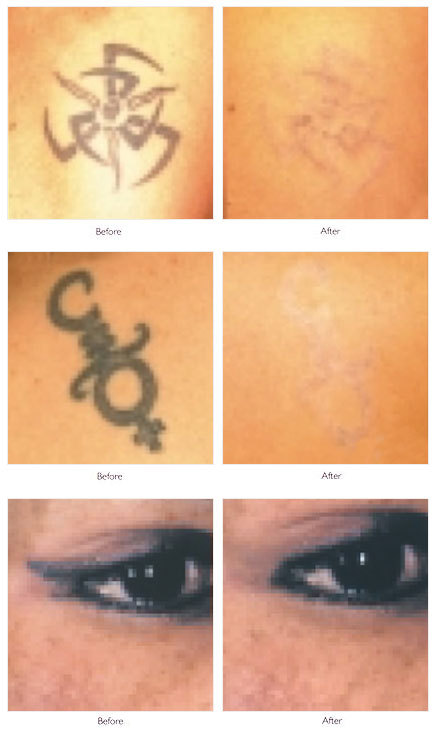
Anderson and Parrish's[16] principle of selective photothermolysis revolutionized the treatment of tattoos. They proposed that if a wavelength was well absorbed by the target and the pulse width was equal to or shorter than the target's thermal relaxation time, the heat generated will be confined to the target. To specifically target tattoos, laser wavelength and pulse duration must be chosen appropriately.
In one of the earliest studies, coworkers examined the effects of a tunable dye laser at 3 wavelengths (505 nm, 577 nm, 680 nm) using a 1-microsecond pulse to remove black, blue, red, and white tattoo pigment. They found the threshold dose needed to induce the same histologic changes was much less than that required for the argon laser, and each wavelength reacted only with complementary colors of tattoo pigment. However, despite the short, 1-microsecond pulse, widespread tissue necrosis was observed, and tattoo lightening occurred only as a result of significant dermal necrosis and resultant fibrosis. They postulated that a shorter pulse, in the nanosecond range, would interact best with the micrometer-sized pigment granule's approximate thermal relaxation time.
To selectively destroy tattoo ink, the best wavelength is chosen to achieve selective absorption for each ink color, while minimizing the nonspecific thermal effects from the primary endogenous chromophores, hemoglobin, and melanin. Black ink is the most common color seen in tattoos, followed by blue, green, red, yellow, and orange. More recent tattoos have a greater range of colors, including shades of pink, brown, purple, and fluorescent colors. Reflectance spectra data for tattoo ink colors may help in selecting the best wavelength. Because of difficulties with absorption spectroscopy, diffuse reflectance spectroscopy was studied (unpublished data from the Candela Laser Corporation) to model tattoo ink using 17 tattoo ink colors and 6 variants of black tattoo ink mixed with gelatin. Reflectance spectra were obtained from 450-1000 nm for each pigment to provide information on their absorption properties.
Black pigment absorbs at all wavelengths (having minimal reflectance), and competition from melanin absorption in the epidermis decreases gradually as the wavelength increases. Absorption for blue and green is greatest at wavelengths of 625-755 nm, while red absorbs best below 575 nm. Yellow primarily reflects above 520 nm, orange above 560 nm, and violet is similar to red in its absorption and reflectance properties. Tan pigment absorbs below 560 nm and flesh-colored pigment below 535 nm. These observations for optimal wavelength absorption for different tattoo ink colors were corroborated by Kilmer et al.
Many tattoo inks are a mixture of colors with a wide range of tint (blue, green, violet, orange) and are difficult to classify as a single pigment. When a color is a mixture of 2 or more pigments (1 of which is reflective, 1 absorptive) some of the reflected light from nonabsorbing pigment reaches the absorbing pigment, causing plasma formation. The resulting reaction may affect both pigments because of the proximity of the 2 pigments in the mixture.
Also, note that the differing composition of amateur (elemental carbon) and professional (organic dyes mixed with metallic elements) tattoos may partially explain why the latter responds less favorably. However, the primary difference between amateur and professional tattoos may be the former's usual paucity of pigment granules. Of 25 tattoos studied, 5 of the 8 amateur tattoos were among the 10 with the lowest volume of pigment granules and cleared rapidly. Overall, tattoos responded according to their total pigment volume.